A large portion of human knowledge and science originates in discovering the truths present in nature. This means that finding out the structure and way objects work in nature forms a grand part of human science. For example, the chemical structure of inorganic and organic substances, and how objects behave and the how plants and animals function are good examples of empirical science. They express the laws present in nature.
Perhaps one of the simplest and most general laws of physics present in the exterior world (nature) is the example of the apple dropping from a tree, which appears as a simple and unimportant event. However, if we consider the factors involved in this simple event (that is, general laws like mass, motion, acceleration, and force) and if we consider the general laws of gravity governing this simple event and extend these laws outside our simple laboratory (that is, under the apple tree), and consider them to be incorporated into a law governing the whole earth and world, it will create wonderful and significant results in mechanics.
Therefore, attention to the tangible laws of nature and the expansion and development of these laws from the experimental limits to the limits which make experiments difficult or impossible can be very profitable and weighty for science.
The author aims to obtain a grand and exceptional achievement for theoretical physics by drawing attention to a simple and wondrous law of the exterior world and by its expansion and development. He also aims to illuminate and explain apparently complicated phenomena such as heat, light, and the physical structure of matter, in ways unbelievably simpler and easier than in present theories.
Review of a simple principle
The first lessons of high school chemistry start with a simple experiment:
There is a red powder which, when heated, gives off oxygen and liquid mercury (a liquid silvery metal). Mercury oxide (HgO), or in simpler terms the red powder of mercury oxide, breaks down into oxygen and mercury with the addition of heat. By contrast, under suitable conditions, the silvery metal mercury can be combined with oxygen and the red powder of mercury oxide results. Apparently, this action of decomposition and combination can be repeated without limit, and definitely the same results will be obtained.
The results produced in this simple experiment, that is, the combining of mercury and oxygen, and, conversely, the decomposition of mercury oxide, have explainable characteristics. The event can be summarized as follows:
When two elements, such as the atoms of NaCl, H2O, or HgO, are combinable and whenever the reactants are placed together (are combined), the physical and chemical properties of the two elements in the combined product go into a latent state, and the new substance (the product) appears with physical and chemical properties completely different from the combining elements. This condition is almost always reversible. The combined product can be decomposed by suitable methods and the component elements freed. Each atom of the freed elements will recover its elemental and initial properties.
Numerous examples like this can be mentioned. One other simple example is table salt, which is the combination of two elements, Na and Cl. The physical and chemical properties of salt are in no way similar to the physical and chemical properties of the combining elements, Na and Cl. If table salt is decomposed and the combining elements are freed, the original elemental and initial properties that were latent will be revealed. This cycle will repeat in a new combination. Thousands of examples of other combinations (H2, O; C, O2) can be given.
Up to here, we have looked at the issue of several substances (NaCl, HgO, H2O) from the chemistry perspective. If we want to define the issue from the physical perspective, that is, express the physical combination of mercury and oxygen and the subsequent decomposition, it is as follows:
To explain the combining elemental atoms and molecules from the physics viewpoint, it is necessary to consider the particles of matter. These particles have their own properties, and we need to consider two or more particles next to each other and the properties of combining and decomposing. Their physical and chemicals characteristics are as follows:
-
When two particles, for example Hg and O, or Na and Cl, are placed next to each other without being merged and their elemental properties are tampered with, they create a new mass which has a new identity and personality, from both the appearance and physical viewpoint and also from the chemical properties’ perspective.
If we name the first particle A and the second particle B, the reaction described above becomes A+B→C.
The physical and chemical properties of particles A and B in the product (new particle) C have become latent. The combination product particle, C, has physical and chemical properties completely different and independent from the initial elements. Frequently, such astounding differences between the characteristics of A and B compared with the characteristics of particle C are seen that we should call it an amazing event.
-
The combination product, that is particle C, which has no chemical or visual similarity with the component particles, is reversible. It can be decomposed, and the particles can be separated from each other. Their physical and chemical properties which were latent in particle C will return to their initial condition, C→A+B
This event of combining and decomposing, or placing these particles of matter next to each other, and separating them can be repeated without limit and without any damage to the raw materials, particles A and B. Therefore, it is better to represent the relationship above as follows: A+B→C.
It is evident that up to now we have not said anything new, and almost every student today who is familiar with chemistry knows this simple law of nature, that is, A+B°C. It is rather easy, especially within the scope of combining atoms of chemical elements to produce various substances and solutions, and to follow the change in physical and chemical properties of elements and materials. For example, investigating the physical and chemical properties of oxygen O2 and hydrogen H2 and their product (H2O) is extremely easy to do.
Oxygen is a gas which causes combustion and hydrogen is a gas which burns. When placed together they produce a wonderful substance called water (H2O). Its apparent chemical properties have no similarity or relationship with the component substances. Electrolysis of water returns the constituent particles of water H2 and O2to their original initial identity.
We can express these several examples that have been mentioned, such as HgO, NaCl, and H2O, and this simple and amazing law of nature which we are considering, in a simpler written form as A+B°C or we can summarize it as the law of A&B. In the continuing discussion, we will need to refer to this law.
We will refer to this law so that the reader can remember that whenever two particles A and B (two atoms from two elements that are combinable together) are placed together (combine) the natural physical and chemical properties of these particles in the resulting product will become latent and the new product’s physical and chemical properties will appear. The reverse action is also possible. In the decomposition of the product, the initial particles will recover their physical and chemical properties without losing anything. It is evident that a compound formed from several elements will conform to the same event, A&B. For example, consider the compound H2SO4. The chemical and physical properties of each and every component element of sulfuric acid remain completely latent as long as they are in the compound. In simpler language, the properties of sulfuric acid have no similarity with S, H2, or O2. Whenever these elements separate, they recover their fundamental and elemental properties, and they reappear.
With this introduction and bringing up the simple law A&B we are getting ready to expand and deepen the discussion.
We will discuss a question in connection with this subject: whether the scope of the law (A&B) should be investigated solely in relation to the combinations of atoms of elements and their molecules and the creation of various chemical substances, or not. In simpler terms, can traces of this law be found in other cases or not?
In connection with chemical elements research on the simple law in this regard is very easy and pursuing it is easy. Investigating and evaluating the properties of elements before and after combining is relatively easy. The physical and chemical properties and substances can easily be analyzed.
For example, we are all aware of the properties of the elements sulfur, oxygen, and hydrogen (S, O2, H2). When they are combined (H2SO4), we can investigate and confirm the physical and chemical properties of the resulting product (sulfuric acid). The law (A&B) in this case has been realized and it can be said that one molecule of sulfuric acid has been formed from seven particles combined (one particle of sulfur, four particles of oxygen, and two particles of hydrogen) which give sulfuric acid its special properties. If we separate these particles from each other under suitable conditions, each particle (element) will recover its own initial properties.
Now we turn our attention away from atoms and molecules to sub-atomic particles. That is, to the point where several different particles in the nucleus of the atom are placed near each other and produce the physical and chemical properties of the element. For example, if we consider an atom of oxygen, we can say that 8 proton, 8 neutron, and 8 electron particles are placed together (combined) and determine the physical and chemical properties of oxygen.
Of course, it may by unusual to use the word “combination” in regard to protons, neutrons, and electrons, but it should be remembered that in using “combination” here we mean that whenever two particles placed together in a particle become latent and a new particle is created with new properties and identity, we can say that these two particles have combined. In other words, they have followed the simple law of A&B.
Our information and knowledge with regard to the nuclei of elements has increased a great deal in recent years. At present, we do not intend to discuss these phenomena. We only want to know whether the law A&B has any application to the nuclear particles of elements. If we mention several simple examples, the topic will become clearer: imagine that we are able to easily and, at will, add or subtract the number of protons, neutrons, or electrons in a nucleus, and note the changes.
Let's consider the element nitrogen, which has 7 protons and 7 electrons, and consider its physical and chemical properties. If we add one proton and one neutron to this collection, a new element called oxygen will appear. Its elemental properties are completely different from nitrogen.
So, we can easily draw the conclusion that traces of the law A&B are to be seen. And we can imagine the opposite reaction. This means that if the nucleus of an element is broken, new lighter elements appear. This can be considered similar to the action of decomposition.
Fortunately, we have information available regarding natural radioactive elements, for example the change in the radium atom A=226 and Z=88 which becomes radon A=226 and Z=86, a gas, by emitting an α particle. Knowledge regarding natural radioactivity and the conversion of one element into another strongly confirms the law A&B. There are various and countless experiments using accelerators, various nuclear bombardments with electron particles and accelerated protons, nuclear disintegration using neutrons and deutrons, measuring other particles of the nucleus, simple capturing of alpha particles, capturing alpha particle radiation, or nuclear fusion or fission, such as thermal fission U=235.
All of these studies and discoveries confirm that when particles with special physical characteristics such as protons, neutrons, and electrons in differing shapes and quantities are placed near each other, they create different physical and chemical properties which we recognize as the different elements. We can see the validity of the law A&B and know that it is credible. We believe more in particles like neutrons, protons, and electrons having physical properties and having or not having electrical charges, and we consider them to have special physical properties. But we don’t know anything about their unique chemical properties. In simpler terms, we don’t have enough independent information at hand about the chemical properties of these particles.
We were easily able to follow the validity of the law A&B with sub-atomic particles like protons, neutrons, and electrons and we find the law correct and noteworthy. This means that if we follow the elements on the periodic table and add protons and neutrons to the nuclei of lighter elements, heavier elements with new properties will be produced. This variety of physical and chemical properties can be defined and explained within the framework of the law A&B.
It is wise to mention several examples of the nuclear changes in various elements that have been created artificially and which have been mentioned as observations in atomic physics books:
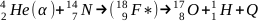
Which is called a complete reaction (a, p). Another example:
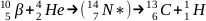
Reaction (a, n)
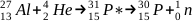
Simple capture of an alpha particle
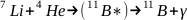
Disintegration from proton bombardment or (P, a)

Disintegration from deutron bombardment

Disintegration from neutron bombardment

If we want to summarize up to now, we can say:
By placing particles of elements next to each other, substances and compounds are produced. The principle of A&B governs the combining of particles and compounds. With regard to various nuclear reactions, by adding or subtracting protons and neutrons in various atoms we can convert them into new elements and still the principle of the law of A&B keeps its validity. However, instead of material particles of elements, we are dealing with material particles called protons and neutrons.
Considering the subject discussed up to now, regarding the application of the theory A&B and the scope of the validity of this law, we can discuss a question under the rubric of where the scope of the law of A&B starts regarding material particles and where it ends.
Following the law of A&B in the compounds of elements is quite simple and easy. For elements to produce a new compound at least two elements are needed to start. In the same way, the participation of several elements yields compounds with larger molecular masses. As we attain massive compounds, we can conceive of the participation of elements in complex and heavy molecules with molecular masses in the thousands.
From the knowledge and experiments in organic chemistry, and about molecules with heavy molecular mass, here is an instructive item on this theme:
When a molecule H2 combines with oxygen and produces one molecule of H2O, since the two material particles of hydrogen and oxygen are almost the same size and mass, a complete A&B event happens. That is, the physical and chemical properties of the two participating particles in the product H2O are now latent. The product compound is basically and fundamentally different in physical and chemical properties from the component particles.
Let us consider a large, heavy molecule of organic matter, such as heavy hydrocarbons, a large polypeptide chain, or molecules of unsaturated fats. If we add a molecule of elemental H2 to these large molecules with molecular masses in the thousands, the small molecule follows the A&B rule completely. This means that its physical and chemical properties become latent in the large body. But there will be no perceptible or significant change in the heavy molecule, that is, no fundamental or measurable change in the heavy molecule. The change in the compound will be very imperceptible and minimal. It is completely natural that in a compound where two particles have great and considerable differences in mass, the smaller particle is subordinate to the larger.
In the compound, its physical and chemical identity becomes latent. The product is the sharing of an exceptionally large particle (big molecular mass) and a small particle with small molecular mass. The small particle causes only an exceedingly small and imperceptible change in the physical and chemical characteristics of the product compound.
This rule about the combination of small material particles with large particles will play an important role in our future discussion. It is a good idea to call it the “rule of disproportionate masses.” This means that whenever we talk about the combination of material particles under the rubric of “rule of disproportionate masses,” it means that if a small particle A combines with a very large particle B in relation to the mass of A, the physical and chemical identity of particle A will follow the rule of A&B, that is the properties will become latent, but the product will maintain its similarity to and the physical and chemical properties of the much larger particle. Any change produced will be minor and perhaps immeasurable.
Fundamental particles
Up to here, the discussion has been more about elements and their compounds and the confirmation of the two laws, or the principle of A&B and the law or “rule of disproportionate masses.” We also found traces of the law A&B in investigating nuclear issues and events. Fortunately, research into the nuclear structure of elements, and work and investigation with accelerators has provided us with important and priceless information, so that we can know about subatomic particles that are much, much smaller than protons and neutrons, and measure and record their properties and characteristics.
In the research on small subatomic particles, they are called fundamental particles in physics books.
We have no intention of questioning the types of fundamental particles and their properties and their role the atoms of elements. The interesting point is when high-energy particles collide with nuclei of elements, they can cause the disintegration of the particles. To make the topic easier to understand, we will give an example:
Let’s consider the atom of an element that has 4 protons and 4 neutrons and the number of fundamental particles is for example 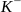 and
and  and
and  and
and 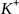 and
and 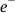 and
and 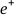 placed next to each other to build the relevant nucleus according to the A&B principle. Until these particles are placed next to each other and create the physical and chemical properties of an element, their individual properties remain latent. If we imagine them in an accelerator and create an enormous collision using high-energy particles such that all the nuclear particles of this atom separate and become independent, then each particle will recover its principle identity and original physical properties regarding charge, spin, etc., We can study the behavior of these particles in detectors and the A&B principle predicts that if one of these particles collides with the nucleus of another atom and is absorbed into the nucleus of the atom (combines again with the nucleus of an atom), its physical properties will become latent and will play its role in the physical and chemical properties of that element in participation with the other particles.
placed next to each other to build the relevant nucleus according to the A&B principle. Until these particles are placed next to each other and create the physical and chemical properties of an element, their individual properties remain latent. If we imagine them in an accelerator and create an enormous collision using high-energy particles such that all the nuclear particles of this atom separate and become independent, then each particle will recover its principle identity and original physical properties regarding charge, spin, etc., We can study the behavior of these particles in detectors and the A&B principle predicts that if one of these particles collides with the nucleus of another atom and is absorbed into the nucleus of the atom (combines again with the nucleus of an atom), its physical properties will become latent and will play its role in the physical and chemical properties of that element in participation with the other particles.
In simpler terms, if a particle is captured in the nucleus of an atom, and if the particle has considerable mass and is determinative such as P proton or N neutron, it can create a basic change in the target nucleus and change it into a new element, that is, change its physical and chemical properties. However, if the particle captured in the nucleus has a small mass and plays a small role according to the "rule of disproportionate masses" the identity of the target nucleus will not change basically or significantly. The small particle in the new nucleus will keep its physical properties latent (until for whatever reason it is freed and recovers its natural properties again).
It must be mentioned that due to their small mass and properties, we cannot find traces of particles or determine their properties in detectors. In other words, component particles of elements in nature are not obliged to be within the scope of our characteristics or within the sensitivity of the detectors. That is, they can be so small that tracking them is impossible. But, these same small particles follow the principle of A&B, and the same "rule of disproportionate masses" is active for them. Therefore, the scope of the A&B principle starts with the smallest material atomic particle and continues up to the compounds of elements, substances, and large molecules. In collisions of accelerated particles with the nuclei of elements, in most cases along with the appearance of changes in the nucleus and release of fundamental particles, a small amount of heat is released in the reaction. The origin and source of this heat can be the preface to a more extensive discussion and the basis of a newer theory about heat. This means, just as according to the A&B principle, very small particles in collisions with accelerated particles with the nuclei of elements are freed and recover their physical and chemical properties. We can construe the heat as very small particles which as long as they are combined in the body of elemental atoms, their identity is latent. If, for whatever reason, these particles of heat are freed, they recover their natural identity and characteristics, which is that same thermal property coming out.
In most chemical reactions, the release of heat or the need for heat to carry out a chemical reaction is known. It would be better to continue the discussion on heat in a separate section and more extensively as to how it follows the principle of A&B and the “rule of disproportionate masses.” Can the present theories best explain the phenomena of heat production and thermal effects or not?
Heat from the perspective of material particles
We will briefly address the theory and conditions for a better understanding of that topic, and which we will investigate it as much as possible to see how we can critique a current theory.
If we want to replace a belief accepted by the majority of minds, what advantages and conditions should this theory have?
In the words of a physicist, a theory always starts with something new, and finishes as dogma. This means, for example, our beliefs with regard to heat start in one place and slowly become firm ideas and beliefs, even part of our scientific faith. Little by little we forget this is a hypothesis or theoretical belief, and it can be correct or not.
Generally, outside of our thinking and minds, phenomena exist in nature. In order to discover the nature and truth in their existence, we are obliged to turn to theories like the phenomena of light, heat, gravity, etc. Because we cannot experiment directly with these phenomena and discover their nature and reality, and because these phenomena are solely in contact with our senses, we are obliged to express the reality of their existence in the framework of one or several theories. In reality, a theory, a hypothesis, our activity of mind, thought, and belief in regard to that phenomenon is special. It must be admitted that a hypothesis will receive more and more acceptance when all the various conditions related to the phenomenon are expressed in the initial thesis. For example, let’s consider light. Various problems in the phenomena of light can be explained in two theories: particle and wave. If one theory could explain all the phenomena with one primary thesis, it would certainly be preferable.
The situation regarding heat is almost the same. A primary thesis should be able to explain all the various conditions of the phenomena related to heat and provide satisfying answers to all the questions. It is wise to mention a topic regarding the evaluation and judging of a new idea and hypothesis, and recall some points. Subconsciously, the beliefs and opinions present in our minds completely interfere with investigating and judging a new idea. Many times they keep us from having independent thoughts and correct and fair judgment on a new thought. Perhaps this condition is natural to a degree. We always judge with our mental tools. But it is suggested that with practice we can strengthen our independence of thought and be more selective in accepting or rejecting a theory. Let’s remember to confront phenomena in such a way that we don’t forget this principle: Theory → Experiment plan → Result of experiment.
Investigation and evaluation of an idea
The researcher should constantly move between the hypothesis and the events of the phenomena. He should juxtapose the various experimental data against the hypothesis and theory, and search for agreement between them. He should not forget that the idea and theorizing might be wrong.
Heat
To investigate the theorizing on the nature of heat and the explanation of its known phenomena, it is better to start with today's theses on heat, and see how the important phenomena are rationalized. Whether a new hypothesis can have preference or not against present theories, it is evident that if a hypothesis is weak and incorrect, no doubt it will be unable to explain the phenomena, and will not answer many of the questions.
Today, the basic hypothesis is that heat is a form of energy, and it exists in two distinct forms—radiation (electromagnetic waves) and molecular motion—in matter. It tries to explain heat phenomena by these two forms of energy. Electromagnetic waves play a role in the generation of heat by thermal displacement. In matter, these waves convert into molecular motion energy (mechanical energy). Conversely, molecular movement in hot bodies can convert into radiation (electromagnetic waves) and transfer heat from one point to another.
Perhaps a big fault and problem in this first step presents itself. The present theories on the mechanism of molecular movement and its conversion to radiation are silent, and don’t give a satisfactory explanation. In other words, how does molecular motion convert its mechanical energy to heat waves? It must be mentioned that molecular motion, especially in gasses and thermodynamics requires several presuppositions (head on collisions of the gas molecules, and the absence of friction between the molecules) which are removed from classical physics beliefs. If, however, we accept that heat is composed of small particles of matter and is subject to the A&B principle; with this one thesis, the phenomena of heat and the effects arising from it are explained.
The theory of particles of heat as a form of material particles is a phenomenon that we call heat. They are formed from very small material particles which as long as they are free can show their physical and chemical properties. This means that these particles can move with the speed of light and can traverse the distance from the sun to the earth. These particles produce a heating effect on detectors. They create the feeling of warmth and temperature in our bodies and they can be absorbed by various bodies and show the effects of heat. They have similarities to the properties of light, such as speed, reflection, and refraction. They have a special place in the light spectrum and special characteristic in crossing translucent media. For example, when we place a vessel filled with water on a burning stove, thermal particles are absorbed by the water (captured between the molecules of water) and causes its temperature to rise. If the vessel with boiling water is placed in a colder environment, the thermal particles will slowly exit (radiation) and disperse into the surrounding environment. These particles also have chemical properties, and in many chemical reactions cause the formation of chemical reactions. According to the principle of A&B, when these material particles are combined with other matter, their physical and chemical properties become latent and their thermal properties will not appear until, for whatever reason, they become free particles and separate from the compound. Therefore, two conditions for thermal particles can be conceived of. One condition is when these particles exist free in nature when they can show their heat and transfer from point to point. The second condition is when particles are combined with elements and other matter. In the second condition, according to the A&B principle, the properties are latent in the matter or elements. In simpler terms, they are saved as stored heat in matter.
Continuing the discussion, we turn to the phenomena which creates heat, and we compare the mentioned hypotheses in the production of heat with the new hypothesis which considers heat to be particles. We will attempt to make the theory simpler and more harmonious with the laws of physics:
1. Friction: The easiest method of producing heat is by creating friction, by rubbing two bodies together (stirring a liquid or rubbing two pieces of metal together). Present theories state that as work is done in a closed system, for example, by rubbing two pieces of metal together, the work performed converts to molecular motion in the metal and produces heat. By performing work, heat can be obtained from this system without limit, and without any change in our system.
On the other hand, the theory of heat particles being material states there are limitless numbers of particles of heat in the combined form with metals, which according to the A&B principle, says that as long as these particles exist in combination in the metal, the physical and chemical properties of these heat particles will remain latent in the metal compound. Friction will cause the particles to release from the metal compound. In simpler terms, they are freed and according to the principle of A&B, their physical and chemical properties reappear in the recognized property of heat.
The comparison of these two theories with each other poses fundamental and basic differences. This means that the first theory claims that the friction of two pieces of metal can produce heat without limit and without producing any change in the system. The second theory states that as a result of friction, freed particles of heat can leave the system (radiation) and if the action of friction continues for a long time and under controlled conditions, the theory predicts the reduction of mass in the system. Performing such an experiment does not seem to be very difficult. It is necessary to mention that when we heat a body (by flame or friction) we can imagine two outcomes for the heat particles in the heated body. Either they will exit, displace, and be absorbed into surrounding matter and some of the particles can combine in the material body and according to the A&B principle the thermal properties will become latent until, for whatever reason, they become free particles and show their heat properties. It is not inappropriate here to refer to the old caloric theory which could not answer the issue of heat production in the phenomenon of friction. It was criticized by Rumford. It goes without saying that the A&B theory answers the defects in the caloric theory.
2. Combustion: In many chemical reactions heat is produced. The most important one is combustion, which is the combination of oxygen and the production of heat (combustion of charcoal, petroleum, etc.) We know materials that naturally store heat, such as wood, coal, oil, gasoline, alcohol, etc. This stored heat can be used in various ways for warming homes or combustion in an automobile engine. With regard to these materials, we can pose a question as to how can heat be stored in these materials (coal and oil), and how are we able to extract them and by burning them (combining with oxygen) use the stored heat? In chemistry, exothermic reactions are mentioned and the conversion of chemical energy into thermal energy is discussed.
Can mentioning the energy between the chemical bonds be a satisfactory answer? If we consider the combustion of coal, we need to heat the coal up to the ignition level for the bonding to occur between carbon and oxygen atoms. This means that to produce a compound, we need heat and that after the combining a considerable amount of heat is freed. What is the origin of this heat? By accepting the heat particles, the issue of combustion and production of heat is simply explained. Let’s suppose that a carbon atom before combining with oxygen has a considerable amount of heat particles according to the principle of A&B in the form of heat particles with their latent physical and chemical properties combined in the carbon and existing in the body of the carbon atom. When one atom of carbon combines with one molecule of oxygen and CO2 is produced, the heat particles in the compound are surplus to the needs of CO2 and are freed. When these particles are freed, according to the A&B principle, they will recover their identity and own physical and chemical properties, that is, heat.
After the combination of oxygen and the formation of CO2, it loses the heat particles and we use the physical and chemical properties of the freed particles in the form of heat.
3. Electricity passing through a wire: Electricity passing through a tungsten wire in a lamp creates heat and light. Present hypotheses claim that the motion of electrons in the tungsten wire raises the energy levels of the electrons in orbit in the element tungsten. The excited electrons produce light and heat waves. In other words, electrical energy is changed into heat and light. Based on present theories, if we imagine a tungsten lamp as a closed system, we should be able to get light and heat from it without limit as long as we give it electrical energy. In simpler terms, we should be able to convert electrical energy into heat and light without limit and without any change in our system.
But the life of the tungsten wire is limited. After a time, the physical quality, especially, changes, and even with the unaided eye the reduction in the amount of light can be seen. The heat particle theory explains the production of light and heat in a tungsten wire as a result of electron flow in this way: the electron flow between the tungsten atoms is from the effect of friction pulling and freeing the heat particles and light particles, and as a result produces light and heat. Therefore, it should be expected that after a long time, the tungsten wire will lose mass and the level of heat and light production will decrease, and the physical quality of the tungsten wire with respect to resistance and other properties will suffer changes.
4. Heat from the sun: The sun is the principal source of heat for the earth, and for plants and living things. We know the production of heat in the stars is related to the fusion of hydrogen. The conversion of hydrogen to helium releases energy. In other words, in this kind of reaction heat is produced in the conversion of matter to energy.
The theory of converting matter to energy and its mechanism is unclear and problematic to a degree. Secondly, if we accept that fusion takes place in the stars, the question is how is this controlled and balanced fusion adjusted with the star’s mass? We know that the larger the mass of a star, the more heat and light, and what mechanism is there to prevent a catastrophic chain reaction? We know that the fusion in a hydrogen bomb takes place in less than a second and causes an explosion.
To answer the question about the production of heat in the stars from the perspective of heat particles, it is better to start with the planets. We know well that all the heavenly bodies like the Earth have considerable mass and their centers produce relatively high temperatures. The material inside is molten and is the source of volcanoes.
The production and explanation of heat inside the earth and by extension all the other planets, is not compatible with present theories. At the center of the earth, neither nuclear fusion nor fission takes place. We know that as we approach the center of the earth, the temperature rises gradually until the temperature causes the material inside the earth to melt. According to the heat particle theory, the only acceptable factor which can produce this heat is the pressure of the upper layers on the lower layers caused by the force of gravity. This means the pressure on different materials causes the vibration of the atoms of matter on each other and creates friction. Of course, we have previously discussed the production of heat by friction and the freeing of heat particles. Volcanic eruptions confirm the presence of great pressure inside the earth. Now, let's suppose the mass of the earth has grown over millions of years (with the addition of rocks falling from space) and is 1,000 or 1,000,000 times larger than today. No doubt it will turn into a fiery planet or star with the increase in mass and the increase in pressure inside. It will be able to produce its own heat and radiate light.
Accepting this by way of producing heat and light for stars is very logical and is closer to physical realities. And given that heat and light are material particles, the reduction in stars’ mass is a natural process and acceptable.
5. The production of heat in nuclear fission was discussed regarding fundamentals as to how these fundamental particles come to be and how heat particles are freed. Present day theories consider the heat produced in nuclear reactions to be from the energy present between the nuclear bonds. Again, whether conversion of matter into energy is correct comes up. However, the heat particle theory maintains this truth that in the atom’s nucleus, in addition to the stored fundamental particles there are many heat particles in the compound, and in nuclear fission a great many of these heat particles show themselves in their physical and chemical properties.
Allow us to mention an example. Imagine a piece of copper metal weighing 500 grams on a laboratory table. The principle says that this piece of metal is composed of billions of millions of particles of matter (protons, neutrons, electrons, and…and heat particles, light particles, gamma particles, magnetic particles, and…) next to each other. This compound made up these particles next to each other makes up the identity of the metal copper weighing 500 grams, and reveals physical and chemical properties. The principle predicts that if we were able to separate all the particles participating in the structure of the 500 grams of copper, it would release a hell of heat particles, light particles, gamma particles, magnetic particles, alpha particles, beta particles. Without a doubt, the destructive power of that would extend to several hundred meters. If we want to summarize the production of heat, it would be as follows: Free heat particles are provided for us by a large source called the sun. The second form is in the form of various substances in a stored form. Any time using a particular method we can free the particles from the compound according to principle A&B, and we can utilize the heat and thermal properties of these particles.
Physical effects of heat
We imagine that the reader of the A&B principle has accepted that the nature of heat is composed of heat particles. When these particles are free in a reaction, these heat particles (solids, liquids, gasses) create phenomena and effects that we want to discuss. The phenomena under consideration include: temperature, heat balance, heat capacity, specific heat, thermal conductivity, expansion and contraction, pressure of gasses, change of phase such as melting, boiling, vapor, and the function of thermal engines, refrigerators, and chillers.
We aim to express the microscopic perspective of the above phenomena and we will compare the present-day theories (molecular motion, etc.) with the particle theories in the proper place.
Free discussion on heat
Based on what has been said up to now, heat is particles of matter, matter which according to the A&B principle exists in all cases in the stored form and in a latent state, and if, for whatever reason, they are released (friction, chemical reactions, nuclear reactions, electricity) the free properties of these particles will find the opportunity to show themselves, as follows: 1. Creation of the feeling of heat. 2. Displacement of heat particles, similar to photons of light. 3. Reaction with matter, solid, liquid, gas, which we study under the rubric of the physical properties of heat. One of the interesting and astonishing properties of these particles of heat is that in reactions with matter and when they gather in atomic and molecular spaces, the space between atoms and molecules is filled and the volume of the matter under consideration increases. This increase in the specific volume is gasses is intense and considerable. Mentioning an example will clarify the issue. Imagine a closed vessel with two volumes of hydrogen and one volume of oxygen. We know that these 3 volumes of gas will produce 2 volumes of water vapor.
Although the volume of the water vapor is less than the initial volume of gasses, the reaction happens in less than a second and the produced water volume occupies a large space. The reason for this event is completely obvious. After the combination of hydrogen and oxygen, a considerable amount of heat particles is freed. They are located between the molecules of vapor, and while it raises the temperature, it causes an increase in the pressure of the volume of water vapor. Similar to this reaction is a mixture of air and gasoline in a car’s cylinder. The release of heat particles increases the temperature and volume as a result of the pressure of the combustion. As a result, mechanical work is done. We will turn to this in a moment.
Now, imagine that we heat some matter (for example, a piece of metal) and then leave it alone. In this heated piece of metal in which heat particles have been captured in the spaces between the atoms, the heat particles will experience two different outcomes. They can combine into the matter of the metal in accordance with the principle of A&B and the heat properties become latent and stored in the metal. Or, the heat particles can exit the spaces between the atoms of the metal as a result of the exchange of heat and be absorbed into the system at a lower level of heat.
We will continue with the physical phenomena of heat with more explanation. We will try to explain a number of heat phenomena which have more fundamental importance. We will place the phenomena of heat particles side by side and compare the theories of explanation.
Brownian motion
Historically, the observation of random motion of particles suspended in liquids is apparently counted as the first explanation of molecular motion. The level of intensity of molecular motion has a direct relationship with the temperature of the solution. At a stable temperature, the level of molecular motion and the resulting random motion of the suspended particles remains stable and uniform.
However, if we pose the issue from the perspective of material particles, the answer to Brownian motion is as follows. Material heat particles exist in the spaces between the molecules of the solution, and they can move from place to place. The higher the temperature, the higher the number of these heat particles, or the motion of the molecules of the solution gives random motion to the suspended particles. As long as the temperature is stable, the movement of heat particles can provide enough force for the random movement in opposition to the attraction between the molecules and the friction between the molecules. It is obvious that the entry and exit of heat particles happens very easily in a solution. The random motion will continue endlessly. In other words, the molecular motion of the solution is secondary to the heat particle motion.
Definition of temperature
Temperature can be defined simply as the level of the density of heat particles in a body. In other words, all balanced systems have similar densities of heat particles. By way of an example, we can investigate the issue more precisely. Imagine we heat a piece of metal. The heat particles enter the spaces between the atoms of the metal and are evenly spread out with the intensity of the heat. The temperature of the metal rises. Here this question arises: whether the heat particles can enter the nuclear spaces and formative parts, like the spaces between the atoms, and whether the distribution of these particles is completely even with equal density in matter. Certainly, the answer is that later we will get to this and we accept that in ordinary heat (other than plasma), temperature is formed by the density of heat particles between atoms of matter (solids, liquids, gasses). In other words, if a body has more compressed heat particles and a higher density of heat particles, its temperature is higher. This heat density can be considered as a heat potential. It can flow to bodies with lower temperatures, and transfer heat. In these cases, only the high temperature is important and there is no relationship to the heat capacity of both sides. It is evident that the piece of red hot metal can transfer its heat to the ocean.
Amount of heat = calorie
The amount of heat or calories provided to a closed system can be defined as follows: the total amount of heat particles present in a closed system is said to be the amount of heat present in the system. For example, one calorie is the amount of heat particles which can raise the temperature of one gram of water by one degree centigrade. Mentioning this issue is necessary since in connection with modern theories, heat is a quantity which is considered to be a type of energy. Under proper conditions it can be transformed into work and mechanical energy, and its amount is reduced according to the thermodynamic formula:
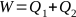 or
or 
Whereas in the heat particle theory, as long as the heat particles present with matter in the system do not form chemical combinations or do not exit the system, the amount of heat is conserved. In simpler terms, here too the principle of conservation of matter governs. The total amount of calories Q in an adiabatic system remains stable. We will discuss this relationship more at later time.
Heat balance: It is natural that in the same way that heat particles are able to penetrate the interatomic spaces of solids, liquids, and gasses, they can exit and move into environments with lower temperatures and create a heat balance.
Thermal conductivity
Physics books define thermal conductivity as follows: the transfer of heat arising from differences in temperature in neighboring parts of a body.
In other words, according to present theories thermal conductivity means that when we heat one end of a body (the temperature goes up), in reality we have increased its molecular motion and this molecular motion transfers to the following layers. In this way thermal conductivity happens, but in practice there are so many obvious disparities in thermal conductivity indices of various solids and gasses that it does not seem to be only an issue of molecular movement. By way of example, if we consider metals, their crystalline and atomic structures are very similar to each other and it is to be expected that electrical conductivity and molar specific heat would be very similar, whereas we obviously know the index of thermal conductivity for silver is 9/9x10, for lead 8/3X10, and for steel 1/1x10. Molecular motion cannot explain the severe difference in the thermal conductivity of these metals. Or, in the case of air which has a very low index of thermal conductivity it would be expected that as we heat some part of the air (we raise its temperature) its molecules which are freely moving would quickly transfer the molecular motion to other parts of the air. However, experiments prove the opposite. When we accept heat as material particles, these particles will have a special connection with reactions and atoms and molecules and various substances, and the behavior of different substances with these heat particles will differ. For example, we know the heat capacity of water is very great, and it does not exchange heat easily with its surrounding environment, and this is related to the special link between water and heat particles. It must be acknowledged we don't know much about this. We will discuss this more in connection with the condensation of gasses.
Expansion and contraction of metals
We know that an increase in temperature causes expansion and a decrease in temperature causes a contraction in the length, surface, and volume in most solids, especially in metals. Present theories believe that vibrations of the atoms in solids (metals) result in the increase in the spaces between atoms as a result of the increase in temperature and the increase in the scope of vibration, and ultimately in the linear, surface, and volumetric expansion of metals. In these theories, if we accept them, at a stable temperature the vibration will continue unchanged without diminishing or stopping (contrary to classic beliefs of mechanics)
At least we should accept that the vibration of atoms is along the X, Y, or Z axes, and if we should heat a piece of cubic shaped metal, cubic expansion will be created. That is, necessarily we must divide the number of atoms into 3 equal parts and believe that each part of these atoms has vibration in each direction of X, Y, and Z. This supposition from the theoretical viewpoint creates a problem, because hypothetically if two neighboring atoms, one in the X axis and the other in the Y axis want to vibrate, they will certainly create a disturbance in linear expansion. We are bound to accept that linear expansion is composed of the algebraic sum of the increase in the vibration of those atoms of metal placed one after another in a straight line.
The expansion of metals in the heat particle theory and the melting of metal
As we have previously indicated, if we construe heat to be particles of matter, these particles can easily enter and exit the interatomic and intermolecular spaces of matter. We said that the greater the density of these particles, the greater the temperature of that body. One of the important properties of these particles is that they occupy space and with increasing temperature they can increase the buildup between neighboring atoms and put pressure on them and separate them. It is evident that the collection of heat particles is predictable and possible in 3 dimensions with axes X, Y, and Z. Thus, they can easily create linear, surface, and cubic expansion.
When the increase and accumulation of heat particles raises the temperature of the metal to such a degree (melting temperature) that it overcomes the attractive force between the metal’s atoms, and separates them from each other, the metal changes phase and becomes a liquid. It is natural that the greater the attractive energy between the atoms in a metal the lower the linear expansion index and the higher the melting point. It seems that when the space of metal atoms reaches a known and common limit, the liquid melting phase (liquid) approaches. The chart on the next page shows that the product of melting temperature of metals in degrees Kelvin and their linear expansion indices yields numbers that in most cases are close to each other. This confirms the existence of a known and common interval for the change of phase from solid to liquid (melting) to happen.
Continuing the discussion of expansion and heat particles
As we previously mentioned, there is no doubt that heat particles can penetrate completely into the bodies of matter (solids, liquids). That is, in the interatomic and intermolecular spaces of matter, and also between the particles and atoms making up the molecules, and the component particles of atoms. However, because of the existence of the strong force between atomic particles (protons and neutrons) at temperatures approaching the melting point of metal and higher than that temperature, they are not able to make fundamental changes in the atomic and molecular structure of bodies, unless at higher temperatures producing plasma where internal nuclear changes in bodies can happen.
Effect of heat on boiling liquids and vaporization
Due to the fact that the intermolecular attraction of liquids is very weak, the change in volume from heat expansion is greater and phase change happens at lower temperatures.
Imagine one kilogram of water in a closed vessel in which the pressure is adjustable for one atmosphere. We add heat until it vaporizes. We want to critique and investigate this process from a microscopic viewpoint according to the molecular motion theory and then from the heat particle theory concept. The molecular motion theory says that the water molecules have weak attraction between them. Each gram of water needs 100 calories of heat in order to reach 100 degrees centigrade. In this interval while the temperature of the water reaches 100 degree Celsius, molecular vibration increases intensely and the water molecules approach the threshold of separating from each other and forming steam. Experiments show that one gram of water which has absorbed approximately 100 calories of heat, absorbs another 540 calories to reach the boiling point, 100 degrees centigrade, (heat is added to it), and change from 100-degree water to 100-degree steam. We call this heat “latent heat of vaporization.” Here we have a question as to what this amount of 540 calories of heat, which for one gram of boiling water is a relatively large amount to change 100-degree water to 100-degree steam, is spent on. In simpler terms, where does this process hide itself? Of course, it is clear that our question includes a microscopic process, and we want to know where these 540 calories fit in the molecular motion theory and what they are used for. This proposition that one gram of 100-degree water takes 540 calories to change from 100-degree water to 100-degree steam is an explanation based on a macroscopic viewpoint of experimentation. From what happens microscopically in the molecules of water, true information is not presented. However, if we follow the issue of boiling and vaporization from the heat particle perspective, it is possible to pursue it.
When we heat water heat particles gather between the water molecules and the water temperature slowly rises until it reaches 100 degrees centigrade (at the pressure of one atmosphere). To simplify and visualize better, let’s imagine that when water is at 100 degrees centigrade 10 heat particles are occupying the space between each two water molecules and have created the relevant cubic expansion. (Opposite diagram)
When these two molecules start to separate more from each other, there is no way the number of heat particles will be enough to keep the temperature of the boiling water at 100 degrees. This is because when the water molecules start to move apart, the density of heat particles, which determines the ambient temperature, decreases. As a result, more heat particles are needed to fill the empty spaces created. For this same reason, a thermometer shows the temperature of boiling water and steam as 100 degrees centigrade. In other words, a thermometer shows the level of the density of heat particles, and in boiling water and steam at 100 degrees the density is equal.
However, the amount of calories consumed for one gram of water at 100 degrees (100 calories) (the total of heat particles) and the calories present in one gram of steam will be 640 calories. It is natural that if this one gram of water which has been converted into 100-degree steam, and occupies about 1670 cc's of space, is transferred to a vessel in which the pressure is less than one atmosphere, it will start to expand (the heating amount is fixed) and the heat particles density decreases and the temperature will start to decrease. Conversely, if we transfer this 1670 cc's of steam at 100 degrees into a vessel and increase the pressure (higher than one atmosphere), the water molecules will become closer and the density of the heat particles will increase. The temperature will start to rise. It is evident that these conceptual experiments should be conducted under adiabatic conditions.
Thus, based on the theory of heat particles the 540 calories that one gram of 100-degree water takes to change into 1670 cc’s of 100 degree steam, is worth investigating and from the microscopic viewpoint is explainable. It is evident that it is presentable as similar to the reasoning on the subject of melting and the heat of melting of metals.
If we heat that same 1670 cc's of steam mentioned above at a constant pressure, the collection of heat particles between the water molecules will increase, and the steam molecules will separate and cause the increase in volume (increase in volume at constant pressure) and if we keep the volume constant and add heat, the space of the steam molecules remains constant, but the density of the heat particles between the molecules rises. If these particles occupy space and cause the pressure on the molecules of steam (increase of pressure in a constant volume) and also cause an increase in temperature. A simple and routine experiment will help in understanding and confirming this issue.
We have all experienced a kettle of boiling water. When the steam exits the spout of the kettle it is hot and can cause burns on the skin. This is because the steam particles are close together and the temperature is close to 100 degrees (at a pressure of one atmosphere). However, if we place our hand or face at a distance of 35-45 centimeters from the spout, the heat will be bearable on the skin because the particles have separated from each other and the temperature drops (the heat particles separate). The drop in temperature in the steam can be felt without a thermometer.
